Introduction
Intramuscular fat (IMF) is one of the most important traits in the meat industry. This adipose tissue is deposited between muscle fibers and it has been positively correlated with tenderness, juiciness of the meat and flavor enhancement (Jung et al. 2016). Beyond a more positive eating experience, meat cuts with high IMF also offer health benefits (Gotoh & Joo 2016), this ensures that they are highly sought after in the marketplace and attract premium prices throughout the supply chain. Even though IMF is a trait that is found in several livestock species (Nurnberg et al. 1998; Kawaguchi et al. 2002; Kirkland et al. 2002), some cattle breeds, such as the Korean Hanwoo and Japanese Wagyu, are renowned for their high IMF content. Breeding programs for these two cattle breeds put a large emphasis on IMF to maintain their world leading position on the meat market.
Multiple methods have been developed to measure the fat content. IMF can be determined by chemical fat extractions (e.g. Soxhlet) and is the most important trait in beef quality; however, marbling score is a visual indicator widely used as well (Sadkowski et al. 2014). Both methods are difficult to accurately measure on live animals which has the obvious downside that if an animal has the desired trait, it cannot be used for breeding anymore. State of the art is still chemical analyses of the longissimus dorsi muscle after the animal has been slaughtered but measures on live animals are improving and a variety of algorithms are available to estimate IMF from CT-scan images (Laurenson & McPhee 2014).
IMF and marbling can be influenced by age (Panjono et al. 2009), feeding time (Carrillo et al. 2016), finishing diet (Carrillo et al. 2016; Kim et al. 2016), gender (Panjono et al. 2009), breed (Wang et al. 2009a; Sadkowski et al. 2014), and it is depot-specific (Lee et al. 2014a). The implementation of specific finishing diets is a common practice to improve the quality of the meat, however, the combination of diets with selection of cattle predisposed to increased IMF are a powerful tool for the beef industry.
Alongside evolving phenotypic measurement techniques for IMF, genomic markers have opened the door to investigate the underlying causative genetic foundations of the trait’s characteristics. Analytical methods based solely on genotypic variations were developed to detected so called selective sweeps or selection signatures. The principle behind these analyses is that populations will evolve and change their genetic patterns to adapt to the environment that they are exposed to or selected for (Amos & Harwood 1998; Decker et al. 2014). With most quantitative traits, the more frequent allele is often associated with the more favorable trait expression and selection leads to an accumulation of this allele. Thus, we observe more homozygous genotypes with the favorable allele, leading to a loss of genetic variation in genomic areas under selection pressure. Applying methods to detect those genomic areas that show a loss of genetic variation are therefore promising to narrow down regions with effects on difficult to measure traits such as IMF.
Other methods combine phenotypic and genotypic information and look for associations between the trait and a genetic marker. With thousands of genetic markers available for the bovine genome, genome-wide association studies (GWAS) have been used to identify regions that harbor candidate genes, e.g. DGAT1 for milk yield or myostatin for double muscling. However, reliable phenotypes are at the core of such association studies. Marbling score and IMF have been shown to have a moderate heritability of 0.31-0.61 which indicates a genetic component in the expression of the trait (MacNeil et al. 2010; Lee et al. 2013a; Mehrban et al. 2017). As such, association studies are a potential way to identify genomic regions that affect IMF. Another form of association studies are gene expression analyses, where the RNA content is measured on divergent phenotypes, in different tissues or at different time-points to identify genes that are active within this tissue or between time-points. This method requires small a amount of the tissue of interest and can be carried out on live animals (via biopsies) or post-mortem.
This review will provide an overview of the main analytical techniques that have been used to narrow down genomic regions associated with IMF, as well as the genomic regions and genes that have been reported for IMF and marbling in Hanwoo. Further, we mention some novel results from signatures of selection, genome-wide association studies and gene expression studies that have not yet been published.
Genetic Background of Intramuscular Fat
The first group of methodologies focusses on genome-only approaches, i.e. where no phenotypic records are used. A drawback of these methods is that any identified region is not necessarily associated with the trait of interest, meaning that any trait under positive or negative selection pressure could be identified. The positive side is that no additional costs for phenotyping occur, especially if the trait is as difficult to measure as IMF.
One of the most popular methods to detect signatures of selection is the fixation index based on allele frequencies (Wright 1951). The fixation index uses the genetic structure of populations to measure differences between them. Within this family of statistics, the FIS value (Weir & Cockerham 1984) determines the loss of heterozygosity within a breed, and the higher the value, the higher the homozygosity of the locus. A study based on FIS values reported 19 unique regions in Hanwoo and 14 in Wagyu cattle (Strucken et al. 2014), of which 2 regions were identified in both breeds (chromosome 11 (40-41.3 Mb), and chromosome 22 (23.4-26.4 Mb). Strucken et al. (2014) also reported 9 candidate genes within the identified regions that could potentially play a role in the expression of IMF (chromosome 1 HGD, PTPLB, chromosome 2 MOGAT1, ACSL3, chromosome 9 ELOVL4, chromosome 16 NPPB and A, chromosome 17 CHD9, chromosome 18 RBL2, FTO). The FST values of the fixation index are based on analysis of variance of allelic frequencies between two or more groups (populations) to test whether the mean values are equal and to what extent there is a differentiation between the groups. Multiple regions have been identified using FST. Porto-Neto et al. (2012) reported 55 genomic regions under positive selection in Hanwoo cattle, of which 24 were unique to Hanwoo. Candidate genes within these 24 regions were associated with immune functions, gamete generation, and fatty acid and lipid metabolism pathways. The latter group included six genes located on chromosomes 12 (CRYL1), 13 (BMP7), 18 (LIPE), and 24 (MC2R, CIDEA, MC5R).
Other methods to detect selection signatures are for example linkage disequilibrium based statistics such as ω-statistic and SFS-based Λ-statistic. A study by Lee et al. (2013b) applied both methods to single nucleotide polymorphisms (SNPs), INDELs, and CNVs in Hanwoo cattle. They identified 3 genomic regions with the ω-statistic (chromosome 18 (5.5-10.5 kb and 15.9 Mb), chromosome 21 (32-33 Mb)), and 2 regions with the Λ-statistic (chromosome 2 (72-72.5 Mb), chromosome 10 (35.5-36 Mb)). None of the genes located within these regions could be functionally associated with IMF based on the current literature.
A third group of analyses is based on haplotypes. Extended haplotype homozygosity (EHH) describes the decay of a haplotype the further away from a core allele it is. EHH values are high if the haplotype diversity is low, thus an indicator of recent positive selection. Similar to the FST value, EHH is calculated between two populations, ideally between an ancestral and a derived population. The integrated haplotype score (iHS) is a standardized measure of EHH. iHS values larger than 2 indicate selection for ancestral alleles. Unpublished data by Strucken et al. calculated iHS for Hanwoo and Wagyu using Buffalo as the ancestral population. They focused on genomic regions previously identified with FIS. Whilst no significant results were found for Hanwoo, three regions on chromosomes 4, 15 and 20 were identified for Wagyu animals. The genomic region on chromosome 15 harbored 49 genes of which 4 genes were associated with backfat thickness in pigs (ACP2), or obesity and adiposity traits in human and mice (MTCH2, NR1H3, PACSIN3).
A variation of EHH is the extended haplotype homozygosity of an individual site (EHHS), which can be calculated between populations but also in a single population (Tang et al. 2007). The integrated EHHS (iES) is the standardized measure of EHHS and was used by Lim et al. (2013) in a Hanwoo population to detect recent selection signatures. They found two genomic regions on chromosome 3 (40.93-43.87 cM) and chromosome 12 (20.79-21.84 cM), which harbored five candidate genes. Three of these candidate genes were directly or indirectly associated with lipid metabolism (DPH5, S1PR1, and CRBN), and could therefore play a role in IMF development.
The Rsb statistic is the standardized log-ratio of the iES and was used by Lim et al. (2015) to identify 16 suggestive regions that displayed Hanwoo specific selection signatures. Within these regions there were 21 genes, of which 14 genes were previously associated with meat traits. In particular the APP gene (chromosome 1, 9-10.5 Mb) was identified as carrying a sequence polymorphism that could explain variation in IMF.
The second group of methodologies uses phenotypes as a direct measure of IMF. Several studies used genome-wide markers to carry out association studies between SNPs and IMF. Using a 10k Affymetrix SNP chip, Lee et al. (2012) reported two regions on chromosome 6 (52.29 cM) and on chromosome 17 (46.04 cM) in Hanwoo cattle, which were significantly associated with marbling. Out of the 10 genes within these regions, one gene (AACS) is associated with fat metabolism and could therefore play a role in IMF expression. In an unpublished study, Al-Mamun et al. carried out a GWAS with imputed sequence data in Hanwoo cattle and identified 17 SNPs that were significantly associated with marbling. None of the genes within the regions could be directly linked to IMF development.
A different approach are gene-expression studies, which use the content of RNA in a target tissue to determine the genes that are active in this tissue and therefore responsible for the expression of tissue specific traits. Wang et al. (2009b) used microarray data to elucidate IMF development. They analyzed differences in gene expression between Wagyu x Hereford and Piedmontese x Hereford crosses as well as biopsies that were taken at 3 different time-points from birth. Ninety-seven genes were differentially expressed both between crossbreds and between time-points. Out of the 6 genes that were differentially expressed and involved in some form of fat metabolism, three genes were also significantly associated with increased IMF (ADIPOQ, SCD, and FAS). Lee et al. (2010) also carried out a gene expression study and equally found a number of differentially expressed genes in a comparison of high and low marbling Hanwoo cattle. They then proceeded to cluster the differentially expressed genes into biological pathways. Three genes (ADAMTS2, CYP51A, and SQLE) were involved in pathways connected to protein catabolic processes and cholesterol biosynthesis processes. The ADAMTS2 gene and a regulatory gene (TGFβ1) were further shown to be associated with increased marbling. In another study, Lee et al. (2011) used 19 markers on chromosome 14 to carry out an association study between markers and different meat traits, including IMF, followed by a gene expression analysis. The LOC614774 locus, located in the previously identified region of 65.585-69.126 cM, was the only locus that was significantly associated with IMF.
A comparison between muscle (MUS) and intramuscular adipose (IMA), subcutaneous adipose (SUA) and omental adipose (OMA) tissues in the longissimus dorsi of Hanwoo showed opposite direction of metabolic regulation between MUS and IMA. Oxidative pathways were upregulated in muscle but downregulated in intramuscular adipose, while collagen, integrin, laminin, fibronectin, and cell junction related genes were up-regulated in IMA (Lee et al. 2014a).
A recent and still unpublished study by de las Heras-Saldana et al. identified 7 differentially expressed genes in Hanwoo cattle with high and low marbling levels. These genes all have ontological function in the regulation of lipolysis in adipocytes (ADORA1, LIPE, PLIN1, FABP4, PIK3R3, PTGER3, PIK3CD).
The FABP4 gene, which is involved in fatty acid transport in muscles, was identified by several previous studies (Wang et al. 2009b; Lim et al. 2015), however, the only study actually testing for an association with IMF did not find significant results (Lee et al. 2011). The LIPE gene was also identified in two studies (Porto-Neto et al. (2012); unpublished de las Heras-Saldana et al.), but again neither of the studies actually tested for a direct association of the gene with IMF. The CAPN1 gene was suggested to be associated with marbling in Korean cattle (Cheong et al. 2008), but a later study by Lee et al. (2014b) was not able to confirm this association. The CAPN1 gene was among a list of genes identified with recent positive selection in Hanwoo, however, no direct link to IMF was established (Lim et al. 2015). Similarly, the CAST gene was previously associated with meat tenderness (Chung & Davis 2012), which is a characteristic for the presence of high IMF, but could not be confirmed (Lee et al. 2014b).
The Polygenic Nature of Marbling
A multitude of genomic regions and genes have been suggested to be involved in IMF development, however, only very few genomic regions or genes have been reported by more than one single study. To some extent, a rationale for these inconsistent findings might be the large variety of analytical methods that were used across the various studies, but even when the same or similar methods were applied, results have still largely remained as singular observations. Another putative reason for the ambiguous results could be due to the phenotypic observations that were used in the association studies. Intramuscular fat can be measured chemically (post-mortem), by CT-scanning, or as a subjective marbling score. Thus, studies might not be looking at the exact same trait. Further, marbling score is the most widely used phenotype for association studies and suffers from being a categorical trait that is subjectively determined.
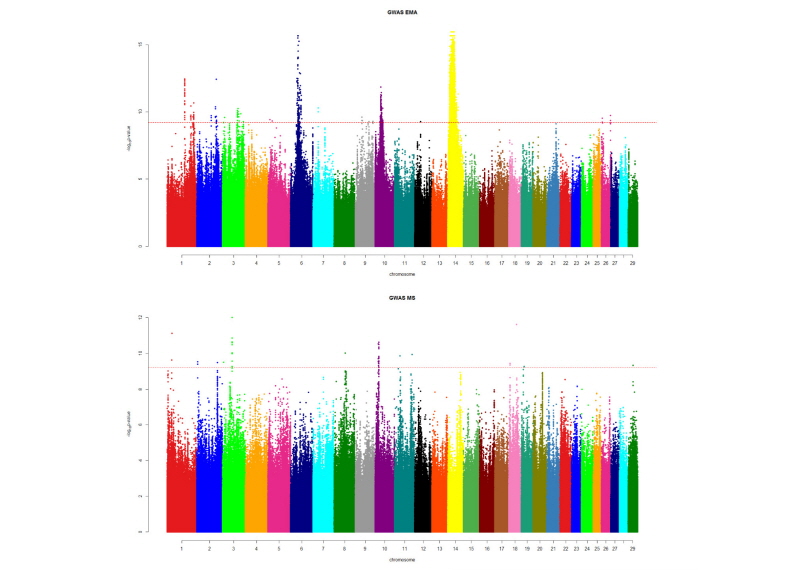
More likely however, even though IMF is a trait of moderate heritability it does appear to be a highly polygenic trait controlled by many genes, most of them with a small effect on the phenotype. Small genetic effects are notoriously difficult to estimate even if phenotypic records and analytical methods are optimal. They also require very large datasets to have enough power to separate true effects from background noise. This polygenic architecture is discernable in association studies with marbling which show relatively unconvincing QTL peaks, especially in comparison to other traits such as body weight that have a few well-known QTL of large effect (Figure 1). The absence of major genes regulating IMF suggests that the lack of reproducibility in IMF studies is, at least partially, due to all the different studies, methods and datasets only being able to discern a small part of the entire picture; each study is partially right, some new facets of this complex trait are unraveled with each new experiment, and probably, some false positives also help to further confound our understanding of the trait.
Conclusions
While the somewhat elusive nature of marbling may be driving researchers to lose their marbles, it is still not an intractable trait. Much has been learnt over the years, quite a lot of this thanks to modern genomic technologies. In practice, industry has and can make good progress in selecting for the trait; but in the short term, it is simpler to treat it as a highly polygenic trait and use appropriate genome-wide selection models (e.g. gBLUP). This does not imply that functional studies for marbling are not relevant; on the contrary, the accuracy of genomic selection for marbling using small marker panels selected based on prior biological knowledge is on par with full sequence data; but it does not do any better either. What this means is that it still will require a lot of research effort and solid financial commitment from industry and funding agencies to advance our knowledge of the true underlying biology of the trait, so that we can finally advance beyond purely quantitative approaches. Future studies will need to be larger and more powerful – marbling is one of those traits worthy of large cross-institutional research consortiums; and highly worth the investment as well, for example, in one of Australia’s high-end steakhouses a Wagyu eye fillet steak rated as 9+ is four times more expensive than a premium Angus steak.
Of course, not only the biology of marbling is important, there is still much that needs to be done in developing more objective methods to measure the trait. Also, marbling and the eating experience are not simply a matter of just increasing the content of IMF; new traits such as speckling (granularity of the marbling) and fatty acid composition play important roles and have not been yet given sufficient attention for breeding purposes. The interplay between marbling and other economically important traits also warrants attention, particularly the relationships between marbling, growth and feed intake, the cornerstones of cost and profit in the Hanwoo industry – we still do not know what genetics are needed to get the highest marbling at the lowest and most effective cost.
In conclusion: much has already been achieved, but a lot still needs to be learnt so that research can continue to deliver valuable outcomes to industry; however, when all is said and done we believe we will find our marble.