Introduction
The diversity of modern horses is rooted in the process of domestication that began 5,000 to 6,000 years ago in the Eurasian steppe. Horses are economically important and popular animal in many industries include agriculture, transportation and recreation (Lippold et al. 2011, Ludwig et al. 2009) Horses are practical animals that breed for endurance, strength, speed and metabolic efficiency (Olsen 2006) and the horses are exceptional model for study of musculoskeletal, cardiovascular and respiratory systems (McCue et al. 2012). The Thoroughbreds is one of famous breeds to use in the horse racing industry.Exercise capacity in Thoroughbred horses have resulted to the emergence of horse industry with worth many billions of dollars (Gordon 2001). Thoroughbred have been selected by breeders to produce a racehorses with the outstanding exercise performance phenotype. Although, exercise and nutrition management have a significant impact on the development of the elite Thoroughbred racehorse, a significant portion of variation in athletic abilities are inherited (Gaffney and Cunningham 1988). Various gene expression occurs in horse skeletal muscle before and after exercise (Eivers et al. 2010). During the development of Thoroughbred, it was confirmed that a genomic region has been selected containing over-representation genes involved in insulin signaling, fatty acid metabolism and muscle strengthening (Gu et al. 2009).
Genome sequencing projects completed in many species have able to conduct genomic selection studies in several species including horses (Venter et al. 2001, Wade et al. 2009). Single nucleotide polymorphisms (SNPs) have been identified in the completed genome-sequenced species. Mostly, SNPs were identified by sequence changes between two alleles (Eck et al. 2009). SNPs are important variations induced phenotypes, traits, diseases (Shastry 2009). Also, SNPs located in the promoter of the economic traits have been related in a variety of morphological traits in horses (Dall’Olio et al. 2012, Dall’Olio et al. 2014).
Genome sequencing projects completed in many species have able to conduct genomic selection studies in several species including horses (Venter et al. 2001, Wade et al. 2009). Single nucleotide polymorphisms (SNPs) have been identified in the completed genome-sequenced species. Mostly, SNPs were identified by sequence changes between two alleles (Eck et al. 2009). SNPs are important variations induced phenotypes, traits, diseases (Shastry 2009). Also, SNPs located in the promoter of the economic traits have been related in a variety of morphological traits in horses (Dall’Olio et al. 2012, Dall’Olio et al. 2014).
In current study, we focused on the characterization of the B3GNT5 expression and genetic variation in Thoroughbred. The expression of B3GNT5 was confirmed in the muscles and leukocytes before and after exercise. Subsequently, the transcription factor (TF) binding sites within the regulatory region of B3GNT5 were predicted. In addition, we analyzed genotype frequency to non-synonymous SNP (nsSNP) in horse B3GNT5.
Materials and Methods
Experimental animals and sample
Blood samples were collected from 98 domestic thoroughbred racehorses that had run a race at the Seoul Lets Run Park. To extract genomic DNA, 900 μL red blood cell (RBC) lysis solution (Solgent, Daejeon Korea) was added to 300 μL of blood, processed for 3 minutes, and centrifuged at 15,000 rpm for 30 seconds. The supernatant was removed, and 300 μL of cell lysis solution (Solgent, Daejeon Korea) and 100 μL of protein precipitation solution (Solgent, Daejeon, Korea) were added, and the solution was processed and mixed thoroughly. The DNA solution layer was collected by centrifuging the solution at 15,000 rpm for 5 minutes, and the supernatant was added to 300 μL isopropanol (Duksan, Seoul, Korea) and shaken slowly. The resulting solution was centrifuged at 15,000 rpm for 10 minutes, and the supernatant was removed. 500 μL of ethanol was added to the supernatant, and the solution was shaken until it became clear and then centrifuged at 15,000 rpm for 3 minutes. DNA was extracted by volatilizing and removing ethanol. (protocol number : PNU-2017-1553)
Polymerase chain reaction analysis
NCBI (http://www.ncbi.nlm.nih.gov) and Ensembl Genome Browser (www.ensembl.org) were utilized to retrieve gene sequence information. The primers used to detect SNPs were synthesized using PRIMER3 software (http://bioinfo.ut.ee/primer3-0.4.0/), and the synthesized primers included B3GNT5 Primer F (5`- CGTGGAGAAGTGTCAAGCAC -3`) and R (5` - TGCAGTTCTCTGTCCTGTGG -3`). To determine the genotype of B3GNT5 SNPs, PCR was conducted on the genomic DNA of racehorses using the following conditions: initial denaturation at 94 °C for 10 minutes; 40 cycles of denaturation at 94 °C for 30 seconds, annealing at 58 °C for 30 seconds, and extension at 72 °C for 30 seconds; and final extension at 72 °C for 10 minutes. PCR products were separated in a 1.5% SeaKem® LE agarose gel (Lonza, Rockland, ME, USA), detected under UV light, and subjected to Sanger sequencing for confirmation after cloning. Cloning of PCR products was carried out using a pGEM®-T Easy Cloning Vector System (Promega, Madison, WI, USA), and each gene sequence was confirmed through Sanger sequencing. SNPs were checked by comparing the gene sequence obtained from sequencing with those obtained from a BLAST search (National Center for Biotechnology Information, Bethesda, MD, USA).
Transcription factor binding site prediction
Transcription factor binding sites were predicted using the ALGGEN PROMO software program v8.3 (http://alggen.lsi.upc.es).
Results and Discussion
Expression of B3GNT5 and B3GNT5-associated genes in horse
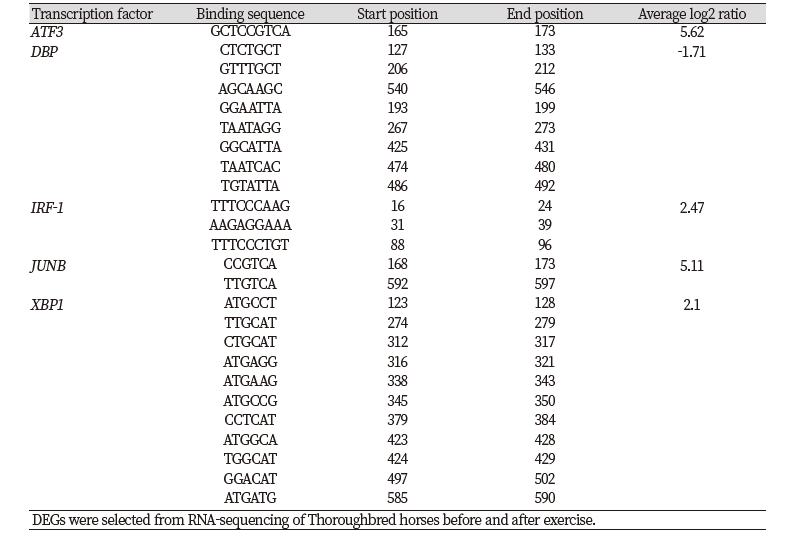
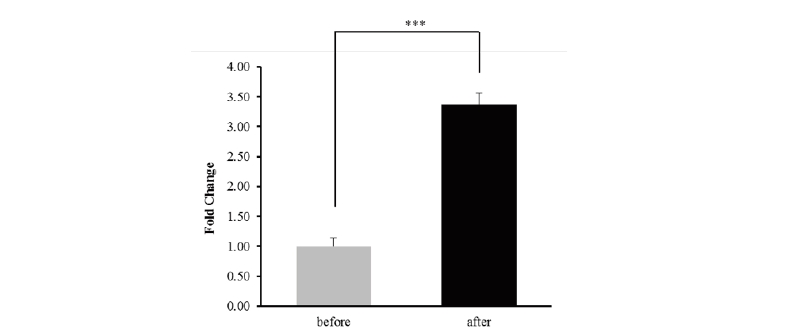
The equine B3GNT5 gene sequence was obtained from whole genome sequencing and RNA-sequencing (Kim et al. 2013, Park et al. 2012). The gene consists of one exon with a total length of 1,128 bp. We found that B3GNT5 expression increased 3.37-fold after exercise compared to before exercise that response to Thoroughbred muscle movement. (Figure 1). To account for this expression pattern, the transcription factor (TF) binding site was predicted up to 600 bp upstream using PROMO in the region defined by B3GNT5 (data not shown). We identified five overlapping genes by comparing between predicted TF and differentially expressed genes. ATF3, IRF1, JUNB and XBP1 were upregulated and DBP was downregulated. (Figure 2 and Table 2).
X-box binding protein 1 (XBP1) is regulates the gene expression of the immune system and important to the plasma cell differentiation (Reimold et al. 2001). B cells that differentiate into plasma cells that secrete immunoglobulin are regulated by the transcription factor XBP1 (Shaffer et al. 2004). B cells affect a variety of immune functions such as the production of immunoglobulins and cytokines (Harris et al. 2000). The cytokines of inflammatory and anti-inflammatory B cells are associated with autoimmunity regulation (Mizoguchi et al. 2002). The expression of JunB proto-oncogene (JUNB) show that increase after acute exercise in humans (Trenerry et al. 2011). JunB protein regulates the expression of cytokines in Th2 cells (Gao et al. 2004). Th2 cells are related to humoral immunity and antibody production (Lakier Smith 2003). Activating transcription factor 3 (ATF3) is induced by a variety of signals, including those initiated by physiological stress and cytokines(Hai 2006). ATF3 acts in a negative feedback system that is induced by many stimuli and limits excessive production of pro-inflammatory cytokines (Hai et al. 2010, Whitmore et al. 2007). Increased levels of Inflammatory and anti-inflammatory cytokines induced by Hard exercise (Pedersen 2000). Cytokines are nonspecific regulated proteins in inflammatory reactions, cell growth and differentiation (Oppenheim 2001). B3GNT5 (beta-1,3-N-acetylglucosaminyltransferase family) is involved in lacto- or neolacto synthesis and carbohydrate structure biosynthesis of HNK-1 and Lewis X especially (Henion et al. 2001, Togayachi et al. 2001). Removal of B3GNT5 in the lacto / neolacto series, which is responsible for Lc3 synthesis expression leads to multiple postnatal defects, including premature death, growth retardation, reproductive and B cell function defects (Biellmann et al. 2008, Kuan, Chang, Mansson, Li, Pegram, Fredman, McLendon and Bigner 2010). Thus, we supposed that B3GNT5 could contribute as an important regulator of inflammatory responses which were caused by exercise.
Association analysis between candidate gene polymorphisms and racing traits of Thoroughbred horses
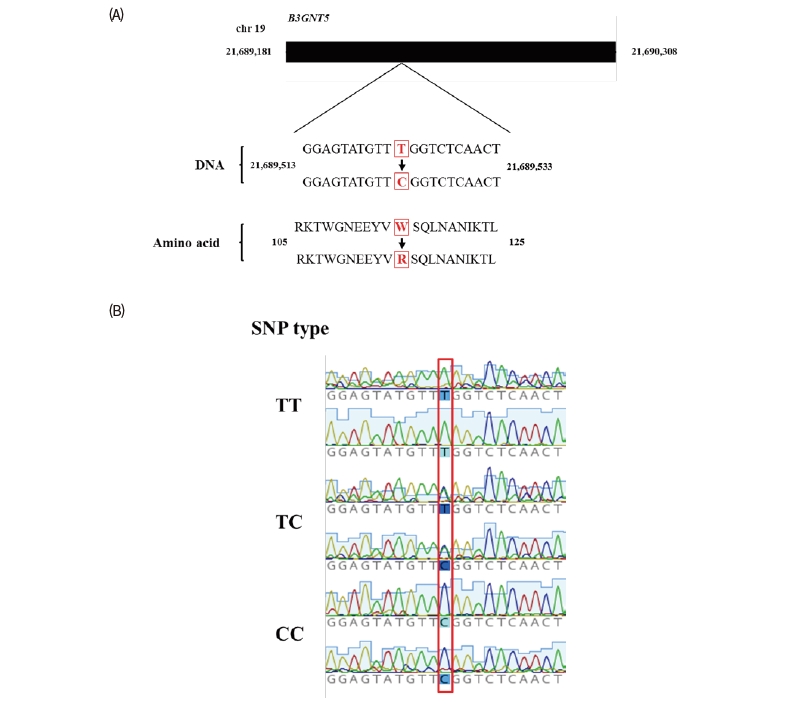
B3GNT5 nsSNP were retrieved from the Ensemble database. The nsSNP (rs69214296) exist the coding regions of B3GNT5 in horse genome and locate in 1st exon (21,689,523th sequence of chromosome 19), where the base change from T to C cause the amino acid change from Tryptophan to Arginine on the 115th amino acid sequence (Figure 3A and B). We identified the frequency of each allele within the racehorse group of known nsSNPs using a blood sample from a race horses. When the genotyping for DNA samples of 98 Thoroughbreds was individually performed. As a result, we found TT, TC, and CC genotype were 4, 33, and 61 respectively and each allele frequency showed 20.92 % and 79.08 % for Tallele and C allele. The Hardy-Weinberg equilibrium analysis was performed to compare the genotyped allelic frequencies with the expected allelic frequencies. An assumption of this analysis method is that the alleles within each subject are statistically independent, at least when no association exists. This is equivalent, assuming that the frequencies of the genotypes in the general population comply with Hardy-Weinberg Equilibrium proportions. The result of χ2 test indicates the significance (< 0.05) between expected values and observed values for the genotypes. As a result, the frequencies of all genotypes were in Hardy-Weinberg equilibrium (Table 2).
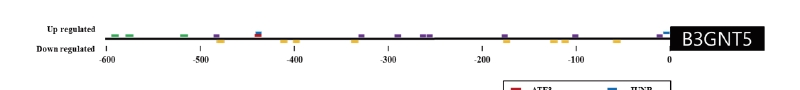
Figure 2. Transcription factor (TF) - binding site prediction in the region from B3GNT5 gene up to 600 bp upstream. The upper element represents the binding site of the upregulated transcription factor and the lower element represents the binding site of the regulatory element in Thoroughbred horses after exercise.
|
Table 2. Chi-squared and Hardy-Weinberg equilibrium test of SNP type distribution for B3GNT5 gene SNP (LOC21689523 T to C) 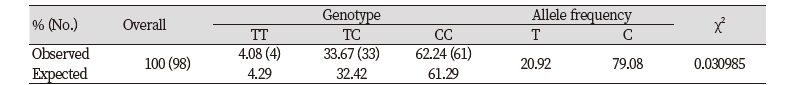
|
Conclusively, we proposed the possibility to correlate between the induction of B3GNT5 and the regulation of inflammatory responses by muscle damages in horse and subsequently nsSNP of horse B3GNT5 and calculated the genotypes in Thoroughbred horse. This study could contribute to future research of the exercise ability of horse.