Introduction
Chicken is the most well-known domesticated animal in the globe. Red jungle fowl (Gallus gallus) is considered as the nearest ancestor of the domestic chicken, which was domesticated about 6,000 to 10,000 years ago in the parts of Southeast Asia (Xiang et al., 2014). Mitochondrial DNA analysis suggests that chicken domestication took place more than 8,000 years ago in the region of Thailand and Vietnam (Komiyama et al., 2004). Chickens were evolved from the therapod dinosaurs (Sereno, 1999) in the medial of the Mesozoic era and have developed individually from mammals for about 310 million years (Bethesda, 2004).
Domestic chickens are not only the cheap source of animal protein to humans, but also widely used as an important model organism in biomedical research (Wu and Kaiser, 2011), connecting the evolutionary gap between mammals and other vertebrates. The chicken is also essential in the field of immunology with the discovery of B cells and medicine with the isolation of the first oncogenes (Brown et al., 2003), and acts as a model for production of vaccine and the study of embryology and development as well as for research on interconnection between viruses and some types of cancer (Bethesda, 2004). The chicken primordial germ cells (PGCs) can be used as a model of choice to establish a new generation of methodology for genome modelling. This PGCs can be used for future chicken genome modelling research in order to study developmental biology, viral DNA-RNA hybridisation, silencing of transgene expression, gene function, epigenetic modification and non-coding RNA (ncRNA) functions (Stupar et al., 2016). Easy entrance to the chicken embryo through incubated eggs and the cosiness of embryo manipulation allows the chick to be an excellent experimental animal for the study of vertebrate development (Stern, 2005). Chicken genome sequence serves as a valuable source of research materials to further increase the nutritional value of poultry meat and egg products.
The chicken represents the first non-mammalian amniotes as well as the livestock whose genome was sequenced (Hillier et al., 2004), leading the way for further understanding of genomics and biology of other bird species. Whole genome sequencing allowed the development of SNP panels, which made high throughput genotyping of hundreds of thousands markers possible (Avendano et al., 2010). Researchers observed that more than half of chicken genes corresponded to indistinguishable human genes. Chicken and human contain the same number of genes, although the amount of DNA is significantly less in chicken than the human genome. Chicken genome contains approximately 20,000 to 23,000 genes in its one billion base pairs of DNA, while the human genome contains 20,000 to 25,000 genes in 2.8 billion base pairs of DNA (Hillier et al., 2004). The sizes of the chicken and the human genome are different due to the inferior numbers of interspersed repeats, segmental duplications and pseudogenes (Hillier et al., 2004).
High-density SNP chips have recently become available for commercial use, allowing the use of genomic information in the poultry industry. However, the poultry genome information had limited accessibility for users, partly because private research interest had incorporated poultry genomics as an option to develop selection and to expand the genetic gain in both broilers and layers industry (Gonzalez- Recio et al., 2009). Nowdays, poultry breeding companies easily access the genomic data for routine use in breeding.
The main goal of animal breeding is to maximize the genetic improvement of economically important traits. Scientists have focused on the development of animal breeding with great interest, after understanding of the structure and purpose of the human genome. The main advantage of genomics in animal breeding lies in the departure from the idea of using data on some genetic loci with a large effect on the traits of interest, moving to using whole-genome information (Meuwissen et al., 2001). Genomic selection in poultry breeding increased the precision of selection intensity, and decreased the generation intervals with better monitoring of inbreeding (Dekkers et al., 2012).
The chicken genetics analysis dates back to the start of the twentieth century. Due to the application of modern technology in the poultry industry, the number of egg production per hen per year increased 3-fold and growth rate 4-fold (Burt, 2002). The development of the genome analysis tools has given entrance to genomics for poultry breeders (Groenen et al., 2011; Kranis et al., 2013). This genomic information could be applied to improve the efficiency of selection by providing a more precise estimation of naturally occurring genetic variation between individuals, and by associating it with traits of economic interest (Wolc et al., 2014). Domestic chickens have the substantial diversity that has been selected for the divergent purpose, as result chickens are used as a model for learning the genetic basis of phenotypic traits (Wong et al., 2004).
The chicken genome database has significantly been developed to meet a large amount of genomic information by applying modern bioinformatic tools (Dhanasekaran et al., 2014). Chicken genomic scientists identified that keratin protein is responsible for the production of feathers, scales and claws in chicken. In very recent years, the transcription activator-like effector nuclease (TALEN) and cluster regularly interspaced short palindromic repeats (CRISPR/Cas9) genome editing tools have been extensively used in the avian systems with genome modification using primordial germs cell-mediation (Lee et al., 2017).
In the last few decades, a significant genetic development for commercially important traits has occurred by selection based on BLUP estimated breeding value in the livestock sector (Hill, 2014). Best linear unbiased predictions (BLUP) has been used in selective breeding based on pedigrees with phenotypes to predict breeding values in poultry (Laughlin, 2009).
Poultry consumption rate increases recently. Also, consumers prefer high-quality products, beinge aware of the food safety, such that there is a demand to reduce the use of antibiotics, chemicals and other different types of growth promoters and additives. Genetic resistance to pathogens in poultry is an important trait, which was hardly performed by traditional genetic selection procedures. However, the solution in poultry sector may be through the application of genomic information (Burt, 2002). In this review, we discuss the application of genomic information in modern poultry breeding sector.
Chicken genome sequence and genome assembly
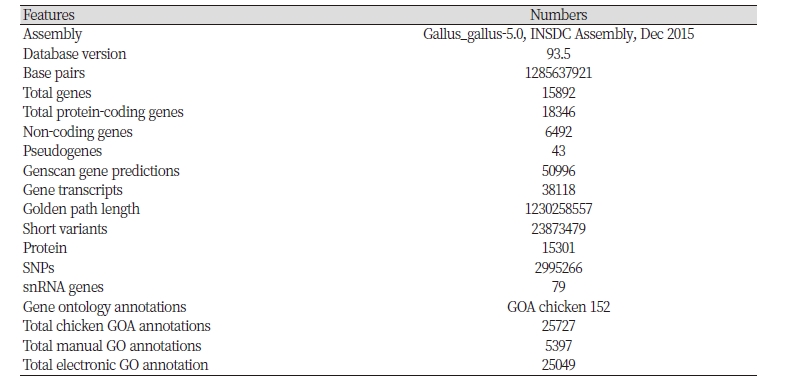
Chicken genome was first sequenced in March, 2004 using a whole-genome sequencing strategy with the assembly of a high-quality sequence (Hillier et al., 2004). Avian genomes have unique characteristics, containing the large variability in chromosome size. Chicken genome holds about 20,000-23,000 genes distributed over their 38 pairs of autosomes (5 macro-, 5 intermediate and 28 microchromosomes) and a pair of sex chromosomes. In chicken sex chromosomes, males are homogametic (ZZ) and females are heterogametic (ZW), but in mammals, males are heterogametic (XY) and females are homogametic (XX) (Bellott et al., 2017). The chicken microchromosomes are gene-rich, higher G+C content, recombination rate and contain at least one-third of the genomic DNA and about twice as genes as the macrochromosomes (Smith et al., 2000). The higher content of G+C is generally connected with the low sequence representation in genome assemblies, which is reflected by the lack of sequences assigned to ten of the 28 microchromosomes in the chicken genome assembly (Eriksson, 2012). Creation of an all-embracing set of DNA probes or so-called ‘landmark probes’ by the individual microchromosomes or the isolation of genomic clones mainly in the bacterial artificial chromosome (BAC) is specific for each chicken chromosomes (Masabanda et al., 2004). Wallis et al., (2004) reported that a BAC map with 20-fold 91% of the coverage of the chicken genome has been congregated into 260 contigs. The chicken BAC has been established from the White Leghorn line (Crooijmans et al., 2000) and an inbred line of Jungle Fowl (Lee et al., 2003). The repetitive DNA comprised only 11% in chicken, whereas mammals contain 40-50%, which was the major contributing aspects to the quality of the ultimate assembly. The crossover rates of chicken macro- and microchromosomes are 2.8 cM/Mb and 6.4 cM/Mb, respectively in contrast to 1-2 cM/Mb for human chromosomes, making the chicken excellent for genetic linkage research. Some traits are controlled by more than one genes in chicken such as a sex-linked dwarf gene. The trait-genes that control the quantitative traits are lcoated at quantitative trait loci (QTL), which can be placed in the genome through association analyses between performance and inheritance of genetic markers in a suitable pedigree (Hillel, 1997). The main factor of the process is a map of genetic markers evenly spaced throughout the genome (Georges and Andersson, 1996). Information from the whole genome are also used to estimate the breeding value (Meuwissen et al., 2001). The use of the DNA markers enabled to estimate breeding values of selection candidates, as soon as DNA samples of animals have been genotyped, which is a main advantage of genomic selection in poultry industry. Expressed sequence tags (ETS) resources are used as a raw material to make cDNA microarrays to research high throughput gene expression (Burnside et al., 2005; Ruby et al., 2006), and also used as gene predictions based on the genome sequence with the use of DNA chips and oligo arrays for the studies of the whole genome gene expression. The genetic and physical maps of the chicken 32,767 supercontigs were assembled into a scaffold of 89% of the estimated 1,050 Mb genome (Burt and White, 2007). The chicken assembly and gene annotations are shown in Table 1. Warren et al., (2017) found a new version of the chicken genome assembly (Gallus_gallus-5.0; GCA_000002315.3) that were assembled from combined long single molecule sequencing technology, improved physical maps, and finished BACs.
Genomic selection in poultry
Genomic selection gives a great impact in the animal breeding industry. The genomic selection was firstly applied in dairy cattle breedings programs (Wolc et al., 2016). In the early stages of the poultry breeding program, selection was mainly on the basis of own performance as well as phenotypic selection. The tradional method was only effective for high heritability traits such as body weight that are estimated in all selection candidates. However the method is not suitable for sex-limited traits such as egg production and quality, and less appropriate for low heritable traits such as disease resistance traits (Wolc, 2014).
Genomic selection takes advantages to statistical models to predict genomic estimated breeding values (GEBV) based on genome-wide single nucleotide polymorphisms (SNPs). The more benefit of GEBV comes from increasing accuracy of GEBV, rather than reduced generation interval, because the generation interval of chicken is short.
The accuracy of genomic selection depends on different factors such as the linkage disequilibrium (LD) between markers and quantitative trait loci (QTL), the size of the reference population and the genetic properties of traits such as heritability or distribution of gene effects (Weng, 2015). The basic objective of the genomic selection is genetic improvement through more precise selection of individuals for better breeding next generations. However, because breeding goals mainly depend on the industry, careful application of genomic information is need in view of genetic gain and profit (Jonas and Koning, 2015).
Development and commercial use of high-density SNP chips enabled genomic selection in poultry breeding. Because high-density SNP chips provide massive, rapid and economically suitable genotyping, genomic selection is currently implemented by all most all poultry breeding industry (Aviagen, 2012; Cobb, 2012; Hy-Line International, 2013; ISA, 2013). Even if the cost of genotyping is decreasing, it still remains one of the main challenges limiting the common application of genomic selection to poultry breeding.
To decrease the cost of genotyping, a small number of major animals can be genotyped at high density and then, imputation can be made for the remaining animals that are genotyped with a lower density panel to obtain high-density genotype data (Heidaritabar, 2016). Aviagen and Cobb Vantress first started the application of genomic selection in broilerswith a 600k SNP chip (Kranis et al., 2013). In 2015, the first descendants were generated from the selected birs with genomic selection and entered into the market as commercial birds (Wolc et al., 2014).
Genomic selection is being applied in other poultry breeds. The turkey genome sequencing (Dalloul et al., 2010) helps to implement genomic selection to achieve faster genetic development for the turkeys. Aslam et al., (2012) reported that the identification of 5.9 million SNPs by evaluating 11 lines of turkeys including 7 lines from commercial breeders.
Non-additive models (Zeng et al., 2013), Bayesian single step (Fernando et al., 2014), haplotype and QTL models (Sun et al., 2014), copy number of variation (Zhang et al., 2014) and genotyping by sequencing (Gorjanc et al., 2015) are new models used in poultry genomic data. However, the accuracy of the selection is difficult in terms of gene flow, i.e. moving genetic progress from elite populations down to commercial populations (Avendano et al. 2012).
Genomic selection is now a major part of regular assessments for broilers and expanded into other poultry species. Application of genomic selection for poultry breeding programs for future research will be highly demandable in the commercial private poultry breeding companies.
Recent status of the poultry genomics
Genomics is a fast-growing and dynamic area in poultry breeding. Application of poultry genomics will be fundamental in allowing the production of poultry meat and egg sectors to meet future growth targets. Isolation and mapping of genetic markers, QTL mapping, candidate-gene identification and gene discovery are the main areas of poultry genomics (Burt, 2002). The current status of chicken genomics will be evaluated with the projections for it's near and long-term future (Dodgson, 2003). Whole genome selection is the latest technology applying the poultry breeding sectors for the development of livestock species (Fulton, 2012). Whole genome sequencing and comparative mapping strategies simplify not only the complex evolutionary trait selection, phylogeny and conserved the gene area of modern domestic chicken (Dey, 2017) but also allowed more precise QTL mapping spanning over various chromosomes and genes for the selection of economically important traits (Hillier et al., 2004; Rubin et al., 2010).
The application of next-generation sequencing technique is widely used in molecular biology and animal genetics research (Dunislawska et al., 2018). Newly discovered 57,636 SNP markers by resequencing of the chicken genome, 328 SNP markers were mapped to microchromosomes and used to build a DNA microarray and detect polymorphisms (Groenen et al., 2011). Poultry breeders have exploited highdensity SNP panels to understand molecular basis of quantitative variation in commercial populations.
In 2005, the Aviagen developed its first SNP panel and identified structure of linkage disequilibrium (LD) in broiler and layer populations (Abasht et al., 2006; Andreescu et al., 2007). Powell et al., (2008) reported that Aviagen also improved the SNP panels to involve the results of whole genome associations in the field of economically important traits. The 60k Illumina SNP BeadChip was constructed to include SNPs known to be segregating at high to medium minor allele frequencies in the commercial broilers and layers (Groenen et al., 2011). The SNP panels with >500k SNP content was also developed by EW Group in both broilers and layers, which was based on the whole genome sequence information for most phylogenetic diversity (Avendano et al., 2010). Resequencing of whole-genome of multiple chicken genotypes has recognized >7 million SNPs (Rubin et al., 2009).
Genomics is also used to identify and remove the possibility of unwanted mutations, to create new traits and to use the improvement of the accuracy of the information generated in the genetic trials. Johnsson et al., (2016) first used a largescale genetical genomics analysis in the chicken brains to identifying genes affecting anxiety as estimated by an open field test.
The complementary DNA (cDNA) sequences are the main component for identification of novel genes and perceiving the complex molecular cascades of organism ontology (Dhanasekaran et al., 2014). The cDNA libraries was formed by suppression subtractive hybridisation (SSH) to detect the genes expressed in the hypothalamus and pituitary associated with broodiness of chicken (Shen et al., 2012), genes expressed in the palingenesis of sensory epithelia (Hawkins et al., 2006), expressed genes in chicken infected with different viral diseases and to study the expression pattern of genes.
Chicken genomics has been also used to identify miRNA related in different pathways and in tissuewide expression (Rathjen et al., 2009). About 496 miRNAs have been mapped and characterized in the chicken genome by cloning, computational vaticination and high throughput sequencing (Dhanasekaran et al., 2014). Solid, Solexa, Ion Torrent and Roche 454 are newly developed tools for the evaluation of expression with enlarged resolution, sensitivity and reproducibility of next-generation sequencing. The Solexa identified 361 novel miRNAs, 88 new miRNA candidates, 33 novel and 189 known miRNAs in the skeletal muscle of layer and broiler chickens in various developmental stages of chicken embryo (Glazov et al., 2008). The chicken miRNA would allow to identify other non-coding RNAs (ncRNA). The chicken ncRNA are used to analyse evolutionary relationships of ncRNA in vertebrates. Gardner et al. (2015) identified 34 IncRNA-associated loci that are conserved between birds and mammals, among which 12 of the loci were validated in chicken.
Applications of the poultry genome information
Chicken and human genomes comparison revelaed that about 75% of coding regions and 30 to 40% of regulatory components are conserved (Hillier et al., 2004). Approximately 2.5% of the chicken genome sequence could be aligned with human, and about 5% of the human genome is under selection, suggesting that the aligned sequences are of functional importance.
One of the main reason of chicken genome sequence is to increase the understanding of the human genome through comparative genomics. Parent-specific gene expression by genomic imprinting is found only in mammals but not in chicken or other lower vertebrates. Comparison of imprinted genes between mammals and chicken open a new window for further research about the features of the origins of imprinting.
Chicken is important amongst developmental biologists, used as a model organism for testing gene function and regulatory sequence. The study on poultry SOX2 genes, for example, neural-specific enhancers were considered by sequence conservation and proved in vivo by electroporation of neural tubes of chick embryos (Uchikawa et al., 2004). The chicken genome information has been used to identify the genes related to the function and regulation of the shell gland, oviduct and egg-shell proteins.
The study of functional genomics help to increase production performance in poultry breeding industry. Prolactin (polypeptide hormone) promoter polymorphism and gene expression regulates the egg production, egg quality, sexual maturity, control the ovarian follicular development and broodiness in various chicken breeds (Cui et al., 2006; Bhattacharya et al., 2011). Chicken genome sequence analysis is now recognizing the extensive set of chemokines, cytokines and their receptors.
The bacterial and viral disease is one of the major threat in modern poultry breeding sector worldwide. Application of the genomic information in broiler and layer strains may reduce the susceptibility of bacterial and viral infection, thus enhancing production capacity. Infection always alters gene sequence and consequently decreases productivity. Salmonella enteritidis infection in poultry persuades the expression of genes related to defence response and cellular communications, while in healthy birds, developmental genes were induced (Tsai et al., 2010).
Genes expressed in various lines can act as a powerful candidate for the identification of genetic markers for Salmonella enteritidis resistance (Chiang et al., 2008). High pathogenic avian influenza virus causes serious economic losses in the poultry industry. To control the outbreaks of the virus, different genomic strategies have been applied in birds to increase the immune response. Chicken genomic microarrays and real-time PCR were used to analyse the immunogenicity of various strains of avian influenza. Genomic information is widely used for the selection of parents and grandparents stock and to identify the vaccine candidates for enhancing immune response and to increase the ultimate production potential of chicken.
Poultry genomics information can be also used in the field of nutrigenomics research for understanding how nutrition impacts the performance, health and disease in poultry. Nutrigenomics is a field of studying effects of nutritional constituents on gene expression (Rawson, 2008). The research of nutrigenomics in the poultry sector will lead to the application of improved precision of feeding strategies. Nutrigenomics plays an important role in poultry production system like the interaction between nutrition and genetics in poultry breeding programs, causing the genetic improvement of performance and increase feed, meat and health quality. It will also regulate the personal nutritional requirements based on genetic makeup as well as the correlation between diet and chronic diseases, which will help to understand the etiologic aspects of chronic diseases such as cardiovascular disease, cancer, type-2 diabetes and the obesity (Reen et al., 2015).
Gene networks could be altered by fasting in post-hatch chicken for a period of twenty-four (24) hours and feed intake restriction, determined by whole transcriptome analysis and associated with energy and hepatic metabolism, cholesterol synthesis and signal transduction in the broiler (Wang et al., 2010; Richards et al., 2010). Nutrition research in the near future will significantly focus on the ways, in which our genes are affected by what we eat and how our animal's genes are affected by what we feed them (Khan et al., 2018). Microarray technology has been significantly applied in the livestock sector as nutrigenomics research for the improvement of food production, food quality and safety in commercial animal industries.
The major challenges for the application of poultry genomic information are incoporation of the genome information into the existing breeding system. Genomic information is still expensive, compared to traditional breeding programs that have huge pedigree records and trait information with predicted breeding values. Therefore, a main successful implementation of genomic information mainly depends on the capability of breeding organizations to assemble the source of information in an optimal way to maximize the accuracy of the estimation of breeding value.
Conclusions
The chicken genome information could create a dynamic path to research in the field of avian biology as well as other animal and plant species. Geneome information is needed to identify genetic variation among the individual and the basis of breed differences such as the difference between layer and broiler lines of chicken. Konweldege of chicken genome sequence is needed to identify the new source of infectious disease in birds and to understand defence mechanism and ultimately to increas performance of production traits. Next-generation sequencing and microarrays are the latest DNA-based technologies used in the poultry sector to study the gene expression. Study of microbial genomics will help to reduce environmental effects and improve sustainability in poultry breeding industry.